Archives > Volume 18 (2021) > Issue 2 > Item 03
DOI: 10.55521/10-018-203
Stephen M. Marson, Ph.D., ACSW, Editor
Journal of Social Work Values and Ethics • Volume 18(2), Copyright 2021 by IFSW
This text may be freely shared among individuals, but it may not be republished in any medium without express written consent from the authors and advance notification of IFSW.
What is IRB?
As a professor emeritus with over 40 years of research experience, the acronym IRB is commonplace jargon for me. In fact, there have been many times when I temporarily forgot what the letters represent. This commonly happens when a clinical social worker who is not involved in research asks, “What does IRB stand for?” My immediate reaction is to explain what an IRB does rather than clarifying the acronym. I continue to forget the R stands for “Review” and not “Research.” Thus, IRB stands for “Institutional Review Board.” The acronym has become a household term among researchers. It is not surprising that a person can forget what it stands for, but not forget what it does.
I know the history: Congress passed the “National Research Service Award Act of 1974” [Public Law 93-348, 93 Congress. 88 Stat. 342 (1974)]. Essentially, the law states, as ambiguously as humanly possible, that if an institution is engaged in human subject research and that research is conducted or supported by a federal agency, then an IRB review is required. For organizations that receive federal funding (i.e., Medicare) for non-research purposes, then an IRB is not required. The question becomes, “What was the catalyst for this Congressional action?”
Why do IRBs exist?
The primary catalyst for the Congressional hearing was the Tuskegee Syphilis Study* (1932-1972). This landmark study, in violating fundamental ethics, is addressed in every social work research text I have seen. In 1932, 600 African American men were selected for a research study. Of these, approximately 400 were diagnosed with syphilis but not told. They were monitored for 40 years. Even after penicillin was available for the general population, the researchers intentionally did not offer the cure to their suffering sample. Subjects were denied treatment because researchers wanted to uncover the long-term impact of syphilis (Laws, 2018). This federally sponsored study was eventually stopped in 1972, not for humanitarian reasons, but because it generated bad publicity. Most scientists were appalled, particularly when they learned that scientists who attempted to complain while the research was being conducted, were censored.
Did we learn our lesson from the Tuskegee Study?
When I was an MSW student at Ohio State University, I experienced an IRB in 1975. I proposed to collect a study sample of persons addicted to alcohol who resided in an inpatient rehabilitation facility. The IRB analyzed my proposal, and I received an oral message (no such thing as email at that time) that my thesis was approved. I did not realize nor was I told that my thesis was being assessed by an IRB. The process was seamless. Almost at the exact time from Ohio State, Middlemist, Knowles and Matter (1976) published their research addressing personal space in public restrooms. Essentially, they monitored the duration and intensity of urine flow in public restrooms in a stranger’s presence. The length of the stranger’s distance predicted the duration and intensity of urine flow. Since Ohio State had an IRB, this research was IRB approved. In a follow-up issue of the same journal, Koocher (1977) condemned the publication of this research contending that it violated the subject protection principles laid out in the American Psychological Association’s Code of Ethics. It is interesting that both my research and their research was IRB-approved about the same time.
The moral of the story is, no matter how much work the IRB does, there will always be research that is approved but remains problematic. The main question we must ask is, what is the proportion of research projects that are approved that should not be? Over 100 years ago, Durkheim specifically addressed this topic with his concept of a community of saints. Within a community of saints, there exists a normal distribution of saintliness. Some saints are saintlier than others. It is reasonable to assume that researchers, like saints, follow a pattern found within a normal distribution. Some researchers are more ethical than others. Thus, we can envision that the ethical procedures embedded within research proposals to be normally distributed. If we employ standard deviations, we can draw the conclusion that problematic research would fall two standard deviations from the mean. As illustrated within the figure, questionable research would constitute approximately 2.5% of the proposals submitted to the IRB.
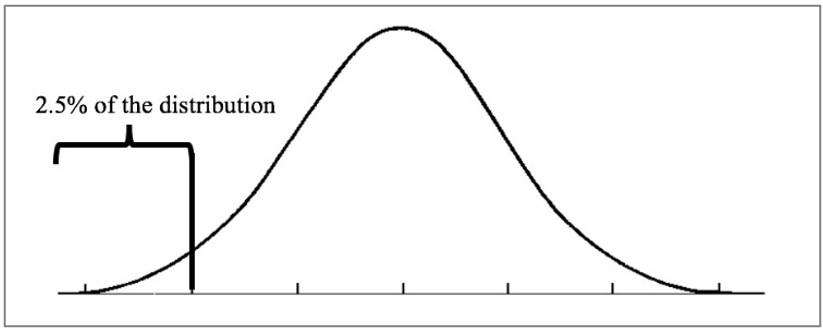
In terms of the millions of research projects seeking IRB approval, 2.5% (25,000 is 2.5% of a million) is a small but substantial number. However, the problem is, members of the IRB must understand that the vast proportion of submissions are going to be presented with unambiguous controls to protect human subjects. IRB members must appreciate and be diligent in their search for only 2.5% of the submissions might be seriously problematic.
What is the implication for 2.5%? In practical terms, highly educated and experienced members of an IRB are feverously searching for aspects of a study that might be harmful to human subjects. In most cases, nothing significant will be found. Researchers who submit to the IRB earnestly construct their proposals to demonstrate that research subjects are free from harm. In terms of the social psychology of board membership, two observations are apparent:
- Searching and consistently finding nothing induces boredom. As a consequence, important nuances of possible harm go unnoticed.
For example, an eminent and internationally respected social work professor of research (in a phone conversation) related this story to me:
An IRB approved a post adoption study in which the central focus was on the satisfaction of the adoption and the adoption process. Even though the IRB and the researcher were diligent in protecting human subjects, a gross error was made. In the process of follow-up, post cards which were IRB approved were mailed to the adopted parents’ addresses. On the post card, an acknowledgement of the adoption was noted. Some of the adopted children were not aware that they were adopted. In fact, they read the post card. This caused serious problems in some of the subjects’ households. The IRB was notified, and the protocol was changed. No members of the IRB were able to predict the serious problems caused by the post card.
- A consistent pattern can lead to uncovering issues that are out of the purview of an IRB. Also, in their frustration of seeking but not finding, an IRB can make grammatical changes in a questionnaire (or other information collection protocol) that stretches subject protection to the point of absurdity.
For example, this happened to a faculty member who was working on a Ph.D. assignment in order to have data to begin a statistical analysis:
As part of a Ph.D. assignment, a faculty member gained IRB approval to submit questionnaires to students in his classes. When he realized that he wouldn’t have enough data to analyze, he set up a booth in the student union and asked passersby to complete his questionnaire. When the IRB discovered this change in the data collection process, they confiscated the completed questionnaires and destroyed all of them. They also destroyed the questionnaires that complied with the original proposal. The questionnaire was copyrighted and purchased to complete the statistical assignment. There was no hearing for the faculty member to defend himself and there was no evidence of potential harm. The chair of the department complained to the Provost who agreed that the IRB was overzealous in their actions. However, since everything was destroyed, nothing was done to compensate the faculty member.
Both examples are products of well-meaning board members who search for potential harm to human subjects and usually finding nothing. A pattern of “finding nothing” can lead to both failures to uncover problems and a false conclusion that human subjects will be harmed when common sense rejects that potential.
The Short Review of Literature
In a brief review of the most recent IRB literature, three patterns were uncovered:
- Support for the current IRB structure,
- Opposition of the current IRB structure (some want to eliminate IRBs),
- Proposed changes to improve IRB structure.
The following table includes recent citations with commentary on IRBs.
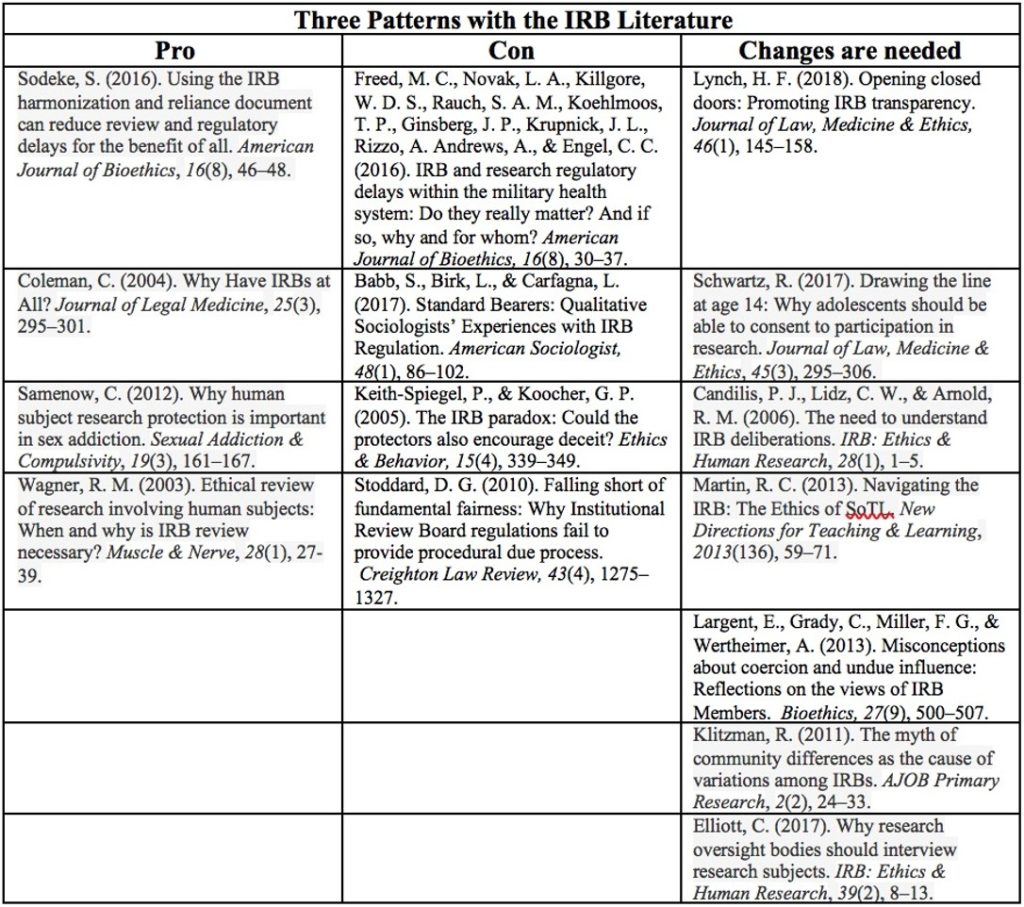
Although the literature fails to address a synthesis of the IRB structure, one can reduce or distill all of the findings to a single issue. An IRB can be no more effective than its weakest member. If a member is “self-impressed,” demands recognition, has an intimidating personality, or is a powerful orator, an IRB may uncover harm to human subjects which actually fails to exist. This causes profound frustration among researchers. In addition, it is clear that some people join an IRB without research experience. They too can cause problems.
The Big Question
Section 5.02 of the NASW Code of Ethics addresses “Evaluation and Research.” If an experienced researcher assesses the expectations of ethical research in the Code, one will immediately become aware that the NASW Code of Ethics is stating “do everything an IRB does.” Essentially, the Code states that social work researchers should conduct a self-evaluation to assure that no harm will come to research subjects. Are self-evaluations effective for uncovering potential harm to human research subjects? Probably not. Research self-evaluation is like becoming one’s own copy editor. Here are two examples of recent proposals in which the researchers were so intensely focused on the methodology, they missed considering the potential harm to human subjects.
- A group of nurses submitted a research proposal addressing new-born circumcision. They proposed to have three groups (two experimental and one control group). The proposed control group was not going to receive a local anesthetic or any other kind of pain reduction protocol. It took less than 30 seconds for all IRB members to reject the proposal as originally articulated. The existence of a control group with no anesthesia was rejected. The IRB members were surprised that the nurses did not recognize the problematic nature of such a control group until after it was explained to them.
- At a large university, a department within the College of Engineering received a large research grant from the chainsaw company. The corporation wanted extensive research on the safety of their newly designed chainsaws. The proposal included a sample of engineering students who would use the chainsaws incorrectly – to assess the safeness of the chainsaws. Here again, everyone on the IRB immediately envisioned the potential harm that could be subjected to paid engineering students who were using the saw in a dangerous manner.
Although the degree of lack of thought was nearly laughable, the two research teams were unable to recognize the potential harm to their human subjects because they were more intensely focused on the methodology and could not see beyond their research goals. It was as if they were wearing blinders. The question is: Is it possible for social work researchers to be so preoccupied with their research question, they fail to consider harm to their human subjects? I suspect that the answer is yes.
Should NASW Require all Social Work Research to Undergo an IRB Review?
Considering a change in an ethical standard that would require IRBs for all social work research cannot be accomplished within a clinical social work paradigm. Research subjects are not like clients. Clients have a plethora of statutes and case law to protect them from a practitioner’s unethical activities. Research subjects do not. For example, to uncover the positive effects of AZT (azidothymidine), the researchers’ goal was to contrast the death count between the placebo and the experimental group. Research subjects who had AIDS signed a waiver which eliminated the ability to gain legal redress for family members. This procedure is the standard research protocol. In addition, there is no state which requires a license for social work researchers. Research subjects do not have the same protection as clients.
Unlike all professional codes of ethics, the ethical foundation of the IRB is proactive. That is, IRB laws are in place to prevent a researcher from committing an unethical act by the actions of an unbiased third party. Unlike the IRB, a violation of an ethical standard is addressed after a violation is committed; the IRB takes action before the unethical act has a chance of being committed. That is because research subjects do not have the protections that clients have.
In making a decision to change the NASW Code of Ethics to require IRB review for all social work research, several facts must be considered:
- There is a difference between research and practice evaluation. Research is defined as a systematic investigation that is designed to contribute to generalizable knowledge. Practice evaluation is traditionally done on a smaller scale and intended to evaluate practice patterns within the institution, not to be generalized across the greater community. Practice evaluation has never been subjected to IRB review.
- There is no professional organization that mandates members of the profession to be subjected to an IRB review. If NASW mandates an IRB review in the Code of Ethics, the organization will be alone.
- If NASW institutes an IRB requirement, only a very small number of social workers will be affected. Social workers employed by universities and large hospitals already face IRB requirements. Those who would be affected would include retired faculty and those who have a private practice or are employed by an agency where research is uncommon.
- NASW has a functioning IRB.
- If the NASW Code of Ethics includes a standard requiring IRB review for all social work research, the rule would impact all social workers. Case law is clear. Once a standard is established within any Code of Ethics, all professionals are subjected to the articulated standards. There is one possible exception. If a state law is contrary to a Code of Ethics standard, then the state law takes precedent. However, this precedent has not been tested in court. Thus, the NASW’s IRB must be made available to professionals who are not members of NASW. Perhaps a fee would be necessary for non-members.
- Can NASW afford the increase in IRB reviews?
Rebuttals
I have shared earlier drafts of this editorial. Therefore, I have the benefit of listening and reading the words of social workers who oppose an IRB requirement. Here is a summary of this material:
- No other professional organization has such an ethical mandate.
My reply: True. The lack of other professional organizations mandating an IRB assessment places NASW in a leadership position. The membership envisions NASW as an organization that emphasizes ethical practice to a degree far beyond other professional organizations. Mandating an IRB for all social work research is uniquely consistent with NASW’s history.
- IRBs slow research findings.
My reply: IRB requirements already exist for social workers employed by universities, hospitals that conduct research and other settings with a formal research component. The IRB requirement would place all social work research on a shared platform.
- When no federal IRB is required, IRBs are unavailable.
My reply: IRBs are everywhere. Most hospitals have them and will accommodate the IRB requests from the outside without a charge. I have requested a hospital’s IRB to assess my proposal regarding online teaching. Their procedure was faster than my university’s IRB. In addition, NASW has an IRB. However, for nonmembers, they are likely to require a fee.
- IRBs are unlikely to find risky research among social workers.
My reply: Yes, this is likely to be true. My best estimate is 2.5% of social work research proposals are ethically problematic. With such a low estimate is it cost effective to require IRB intervention for all social work research? The NASW Delegate Assembly must decide.
I am interested in learning your opinion. Let me know what you think about an IRB requirement within the NASW Code of Ethics. Send your thoughts or commentary to smarson@nc.rr.com.
*If the reader has a CSWE accredited degree in social work (Bachelors, Masters and even doctorate) and has not heard of the Tuskegee Syphilis Study, email me. I will contact your campus and ask that you receive reimbursement for your tuition.
References
Babb, S., Birk, L., & Carfagna, L. (2017). Standard bearers: Qualitative sociologists’ experiences with IRB regulation. American Sociologist, 48(1), 86–102.
Candilis, P. J., Lidz, C. W., & Arnold, R. M. (2006). The need to understand IRB deliberations. IRB: Ethics & Human Research, 28(1), 1–5.
Coleman, C. (2004). Why have IRBs at all? Journal of Legal Medicine, 25(3), 295–301.
Elliott, C. (2017). Why research oversight bodies should interview research subjects. IRB: Ethics & Human Research, 39(2), 8–13.
Freed, M. C., Novak, L. A., Killgore, W. D. S., Rauch, S. A. M., Koehlmoos, T. P., Ginsberg, J. P., Krupnick, J. L., Rizzo, A. Andrews, A., & Engel, C. C. (2016). IRB and research regulatory delays within the military health system: Do they really matter? And if so, why and for whom? American Journal of Bioethics, 16(8), 30–37.
Keith-Spiegel, P., & Koocher, G. P. (2005). The IRB paradox: Could the protectors also encourage deceit? Ethics & Behavior, 15(4), 339–349.
Klitzman, R. (2011). The myth of community differences as the cause of variations among IRBs. AJOB Primary Research, 2(2), 24–33.
Koocher, G. P. (1977). Bathroom behavior and human dignity. Journal of Personality & Social Psychology, 35(2), 120–121.
Largent, E., Grady, C., Miller, F. G., & Wertheimer, A. (2013). Misconceptions about coercion and undue influence: Reflections on the views of IRB Members. Bioethics, 27(9), 500–507.
Laws, T. (2018). Tuskegee as Sacred Rhetoric: Focal Point for the Emergent Field of African American Religion and Health. Journal of Religion & Health, 57(1), 408–419.
Lynch, H. F. (2018). Opening closed doors: Promoting IRB transparency. Journal of Law, Medicine & Ethics, 46(1), 145–158.
Martin, R. C. (2013). Navigating the IRB: The Ethics of SoTL. New Directions for Teaching & Learning, 2013(136), 59–71.
Middlemist, R. D., Knowles, E. S., & Matter, C. F. (1976). Personal space invasions in the lavatory: Suggestive evidence for arousal. Journal of Personality & Social Psychology, 33(5), 541–546.
Samenow, C. (2012). Why human subject research protection is important in sex addiction. Sexual Addiction & Compulsivity, 19(3), 161–167.
Schwartz, R. (2017). Drawing the line at age 14: Why adolescents should be able to consent to participation in research. Journal of Law, Medicine & Ethics, 45(3), 295–306.
Sodeke, S. (2016). Using the IRB harmonization and reliance document can reduce review and regulatory delays for the benefit of all. American Journal of Bioethics, 16(8), 46–48.
Stoddard, D. G. (2010). Falling short of fundamental fairness: Why Institutional Review Board regulations fail to provide procedural due process. Creighton Law Review, 43(4), 1275–1327.
Wagner, R. M. (2003). Ethical review of research involving human subjects: When and why is IRB review necessary? Muscle & Nerve, 28(1), 27-39.